how many valence electrons does nitrogen have
Sulfur can expand its valence shell to hold more than eight electrons but oxygen cannot. A nitrogen atom has seven electrons.

How To Find The Valence Electrons For Nitrogen N Youtube
The radius of a sulfur atom is about 60 larger than that of an oxygen atom.
. Because 5 3 it leaves unused two electrons in a lone pair unless there is a positive charge around like in NH 4. In the ground state they are arranged in the electron configuration 1s 2 2s 2 2p 1 x 2p 1 y 2p 1 zIt therefore has five valence electrons in the 2s and 2p orbitals three of which the p-electrons are unpaired. Nitrogen Group or Pnictogens. To determine how many total electrons there are add the amount of charge to the atomic number.
Nitrogens atomic number is 7 therefore this ion has 10 electrons. When a pnictogen forms only three single bonds effects of the lone pair typically result in trigonal. In this case the valency of the nitrogen atom is 3. Carbon Group or Tetrels.
Counting the total number of valence electrons of one nitrogen atom two oxygen atoms and the additional one negative charge equal to one electron. Xe 6s2 for barium. Group 13 means group 3 so on and so forth. Oxygen Group or Chalcogens.
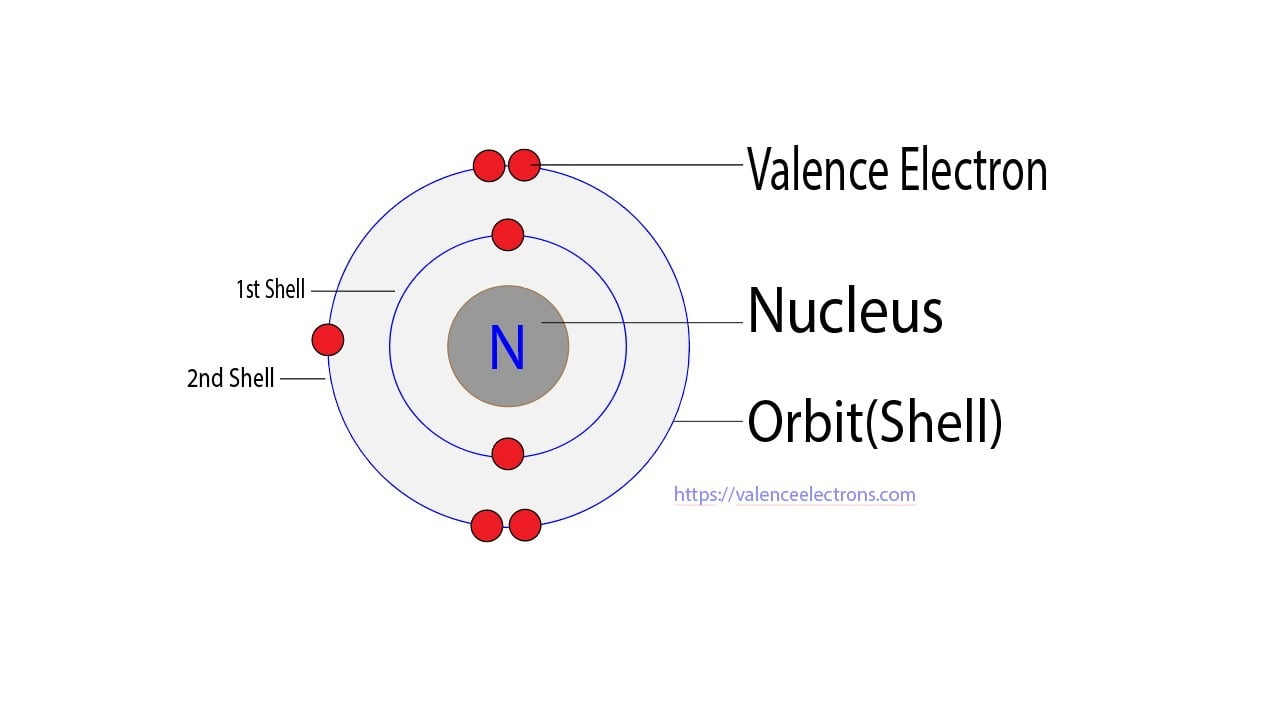
Electrons which are the electrons in the outermost noble are a family of elements that have very little tendency to react. Total Number of valence electrons is. After arranging the electrons it is seen that the last shell of the nitrogen atom has five electrons. Then play a game to test your ideas.
Valence electrons and What I know Solution. The electron configuration can be visualized as the core electrons equivalent to the noble gas of the preceding period and the valence electrons eg. - Group 16 VIA - 6 valence electrons. Which orbital holds up to 6 electrons.
- Group 15 VA - 5 valence electrons. The nucleus is located in the center of the atom. COCl 2 phosgene or carbonyl chloride 24 valence electrons 4 6 2x7 Place the C in the center and connect the O and the two Cls to it. Protons and neutrons are located in the nucleus.
N 2s 2 2p 3 2O 2s 2 2p 4 1 negative charge 5 26 118e Step 2. Oxidation states are typically represented by integers which may be positive zero or negative. By the time we reach the final column of noble gases all the. It also tells us how easily atoms can make bonds how many unpaired electrons there are and how.
In this case there are fewer protons than electrons. There are two oxygen atoms so the total amount of valence electrons in our diagram is. As we move from left to right each column has one more valence electron. Nitrogen is a group 15 element and so has 5 valence electrons while group 16 oxygen has 6 valence electrons.
- Group 14 IVA - 4 valence electrons. For atoms with many electrons this notation can become lengthy and so an abbreviated notation is used. The relative placement of the O and the Cls does not matter since we are not yet drawing a three. Most of these elements have a set of eight outermost electrons which is called a stable 25 Atoms tend to want to have a set of 26 will lose or gain electrons to achieve it.
Notice also that for sulfur this 12- electron stable energy minimum is unrelated to the larger numbers of valence-related electrons seen in transition element shells since sulfur simply does not have enough electrons to access those more complex orbitals. Recognizing Families on the Periodic Table. - Group 18 VIIIA - 8 valence electrons. For the main group and non-metallic elements it is easy to tell how many valence electrons an element has just by looking at the periodic table.
Electrons equal to protons are located. That is the number of protons in the potassiumK is 19. Each nitrogen atom will share 3 of its valence electrons in order to form 3 bonding pairs of electrons a triple covalent bond so that each nitrogen atom will have a share in 8 valence electrons electronic. Since filled d or f subshells are seldom disturbed in a chemical reaction we can define valence electrons as follows.
Drawing a single bond between nitrogen and. Our diagram should have 17 electrons in total. Sketch the atomic structure of the compound. How many valance electrons does sodium Na have and what is its highest energy level.
The electrons on an atom that are not present in the previous rare gas ignoring filled d or f subshells. The module presents chemical bonding on a sliding scale from pure covalent to pure ionic depending on differences in the electronegativity of the. How many valence electrons does potassium K have. Build an atom out of protons neutrons and electrons and see how the element charge and mass change.
- Group 17 VIIA - 7 valence electrons. Now all of the valence electrons have been used up the octet rule is satisfied everywhere and all of the atoms have formal charges of zero. Here you will know about the atomic mass of every element in the table we all know that there are 118 elements in the table and it is one of the important topics of chemistry and there are many students who have to perform practical and in order to perform the practicals one must be knowing the atomic number so that their experiment doesnt fail and complete its objective. Because valence electrons have higher energy than electrons in inner orbits they are involved in the majority of chemical processes.
They assist us in determining the chemical properties of an element such as its valency or how it forms bonds with other elements. In Section 5 we learned that valence electrons are the electrons that dictate atomic behavior. How many electrons and protons does the potassiumK atom have. 51 62 17 electrons.
The 12 electron valence shell of ceSF6 is instead a true bending of the rules for an atom that in nearly all. Each nitrogen atom Group 15 has 5 valence electrons A nitrogen atom needs 3 more electrons in order to complete its valence shell that is to make up 8 electrons in the L shell. The atomic number is the number of protons. The skeletal structure of nitrite ion is written as O-N-O.
Nitrogen N has only five valance electrons because it is in group 5 though it is actually in group 15 you are going to ignore the transitional metals group 3-12 because these groups have different way of determining their valence electrons. The millions of different chemical compounds that make up everything on Earth are composed of 118 elements that bond together in different ways. In this case the valence electrons of. Formation of multiple bonds is facilitated by their five valence electrons whereas the octet rule permits a pnictogen for accepting three electrons on covalent bonding.
This module explores two common types of chemical bonds. Which electron configuration represents the electrons of an atom of neon in an excited state. For example N 3-has a -3 charge which means it has 3 more electrons than a neutral nitrogen atom. The electrons in the outermost shell are the valence electrons the electrons on an atom that can be gained or lost in a chemical reaction.
The atomic number of potassiumK is 19. The Effect of Differences in the Strength of X-X and XX Bonds. It has one of the highest electronegativities among the elements 304 on the Pauling scale exceeded only by chlorine 316 oxygen 344. How many valence electrons does nitrogen ionN 3- have.
These seemingly minor differences have important consequences for the chemistry of these elements.

How Many Valence Electrons Does Nitrogen N Have

How Many Valence Electrons Does Nitrogen Have Quora

What Is The Number Of Valence Electrons In Nitrogen Socratic

Posting Komentar untuk "how many valence electrons does nitrogen have"